
Optical rotation in 5 ɴ-HCI, 0 to +25, respectively (values for glutamine and tryptophan with water as solvent, and for asparagine 3♴ ɴ-HCl) Optical rotation in 5 ɴ-HCl, D 0 to -25, and over -25, respectively Logarithm of solubility in water of the ʟ-isomer in mg/100 ml., scored additively PK at isoelectric point, scored additively in steps of 1 pH Presence, respectively, of: flat 5-, 6- and 9-membered ring system Molecular weight (approximate) of side chain, scored in 12 additive steps (sulphur counted as the equivalent of two carbon, nitrogen or oxygen atoms) Presence respectively of: isopropyl group, distinct aromatic reactivity, strong aromatic reactivity, terminal positive charge, negative charge at high pH (tyrosine scored positive) Presence respectively of: imidazole ―NH group, indolyl ―NH group, ―SCH 3 group, a second optical centre, the N=CR―NH group Presence respectively of: sulphur atom, primary aliphatic ―OH group, secondary aliphatic ―OH group, phenolic ―OH group, ability to form S―S bridges Presence respectively of: ―CH=N, indolyl group, imidazole group, C=O group in side chain, and configuration at α―C potentially changing direction of the peptide chain (only proline scores positive) Presence respectively of: guanido group, α―NH 2, α―NH group in ring, δ―NH group in ring, ―N= group in ring Presence respectively of: benzene ring (including tryptophan as positive), branching in side chain by a CH group, a second CH 3 group, two but not three ―H groups at the ends of the side chain (proline scored as positive) and a C―S―C group Presence respectively of: ω―SH, ω―COOH, ω―NH 2 (basic), ω―CONH 2 and ―CHOH groups Presence respectively of: β―CH 2, γ―CH 2, δ―CH 2 ( proline scored as positive), ε―CH 2 group and a―CH 3 group Properties of amino acids employed for estimating overall similarity Two-state characters Each physicochemical distance is based on different composition of properties. There are 134 physicochemical properties that can be used to estimate similarity between amino acids. The value of distance is based on properties of amino acids. Physicochemical distance is a measure that assesses the difference between replaced amino acids. A well known example in humans is sickle cell anemia, due to a mutation in beta globin where at position 6 glutamic acid (negatively charged) is exchanged with valine (not charged). This can lead to changes in protein structure or function, which can cause potentially lead to changes in phenotype, sometimes pathogenic.

Usually amino acids are thus classified into two types: Similarity between amino acids can be calculated based on substitution matrices, physico-chemical distance, or simple properties such as amino acid size or charge (see also amino acid chemical properties). The magnitude of this process may vary depending on how similar or dissimilar the replaced amino acids are, as well as on their position in the sequence or the structure. Not all amino acid replacements have the same effect on function or structure of protein.
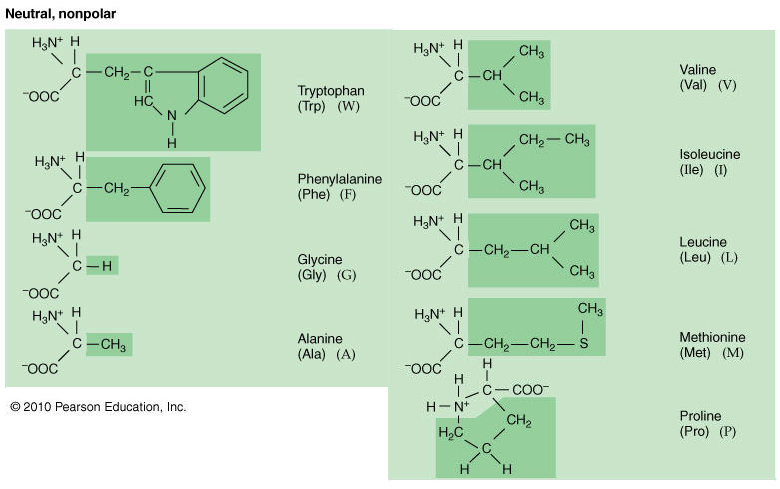
All polar amino acids have either an OH or NH2 group (when in aqueous environment), and can therefore make hydrogen bonds with other suitable groups. The properties of the amino acid are due to the properties of the side chain or R-group. If no partial charges, it's a nonpolar amino acid.
#Hydrophobic amino acids and neutral polar amino acids full#
While this won't tell you if a given atom achieves a full charge, or only a partial charge it's sufficient to let you know if there are atoms in a side chain with partial charges… Which means that side chain has polar elements. Polar amino acids include serine and threonine (contain a hydroxyl group), asparagine and glutamine (contain amide group).Īlso Know, how do you know if an amino acid is polar or nonpolar? These amino acids are usually found at the surface of proteins, as discussed in the Proteins 2 module.Īlso, are basic amino acids polar? The charged amino acids include two basic, lysine and arginine (+ charge), and two acidic, aspartate and glutamate (- charge).
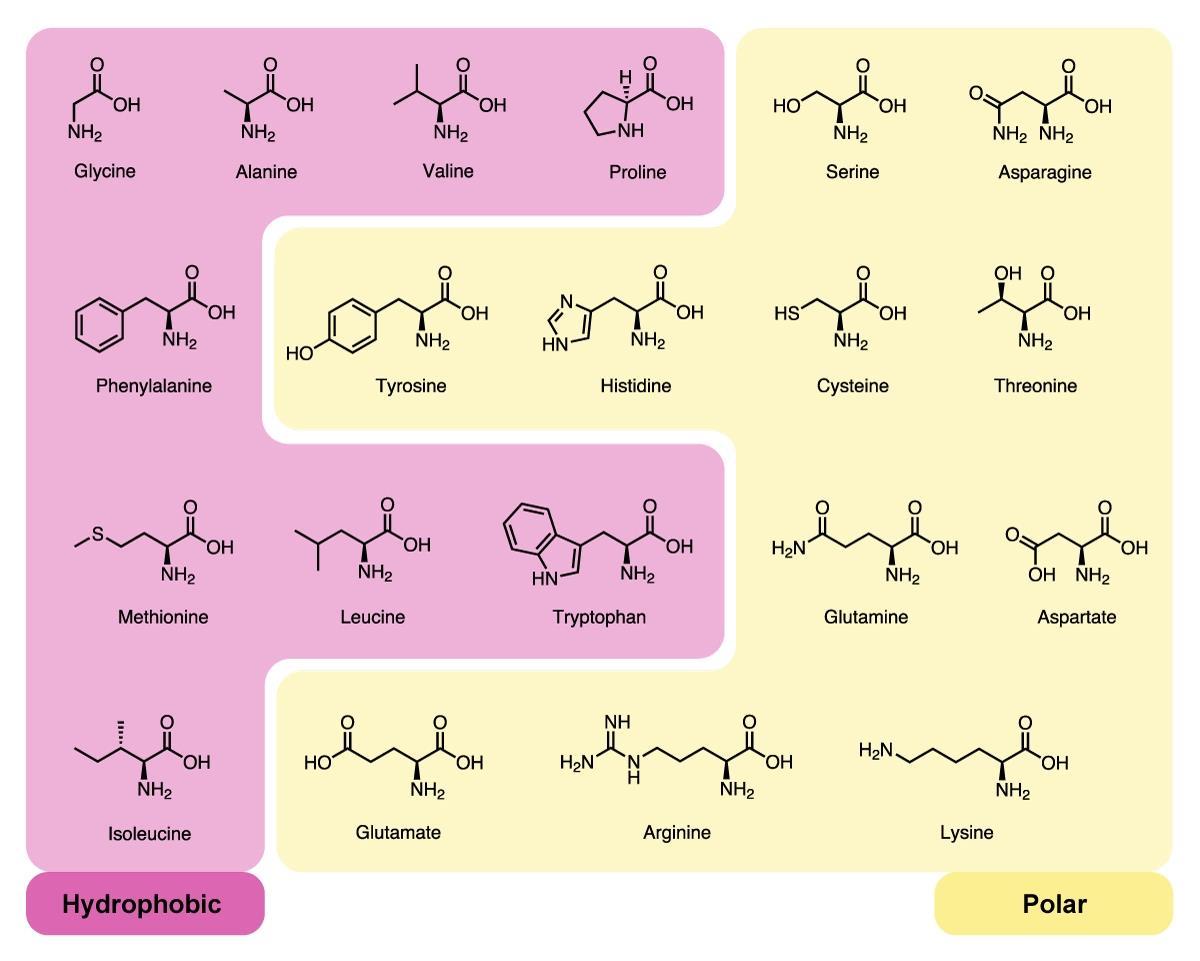
These are serine (Ser), threonine (Thr), cysteine (Cys), asparagine (Asn), glutamine ( Gln), and tyrosine (Tyr). Six amino acids have side chains that are polar but not charged. Likewise, people ask, what amino acids are polar?


 0 kommentar(er)
0 kommentar(er)
